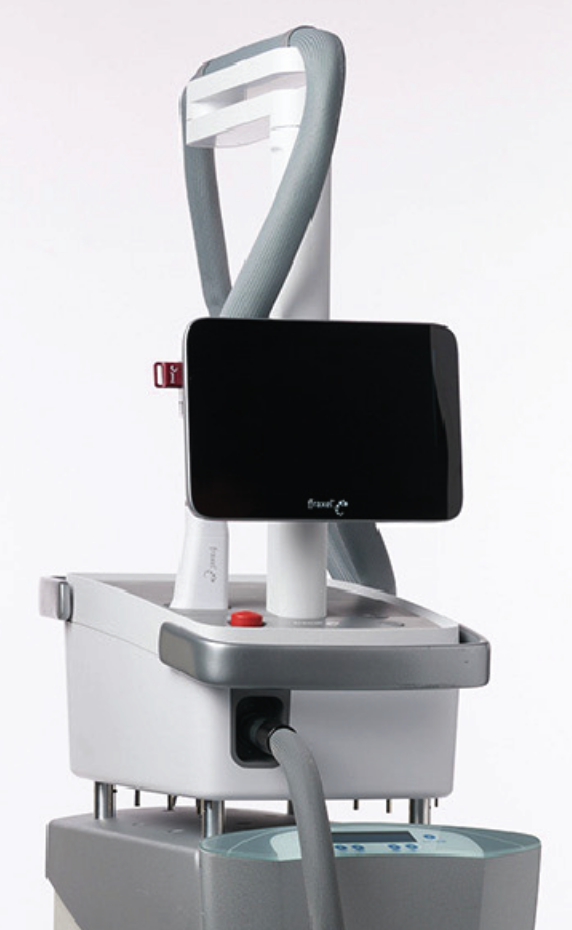
MODERN DESIGN WITH IMPACTFUL CAPABILITY
A VALIDATED TECHNOLOGY
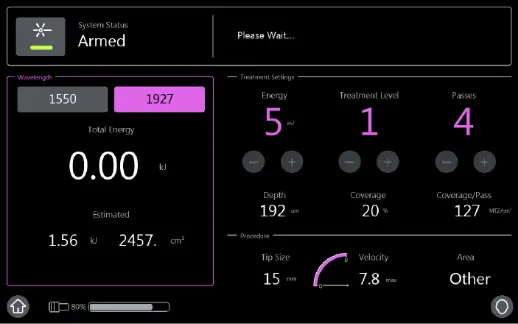
THE FAST TRACKING XPERIENCE
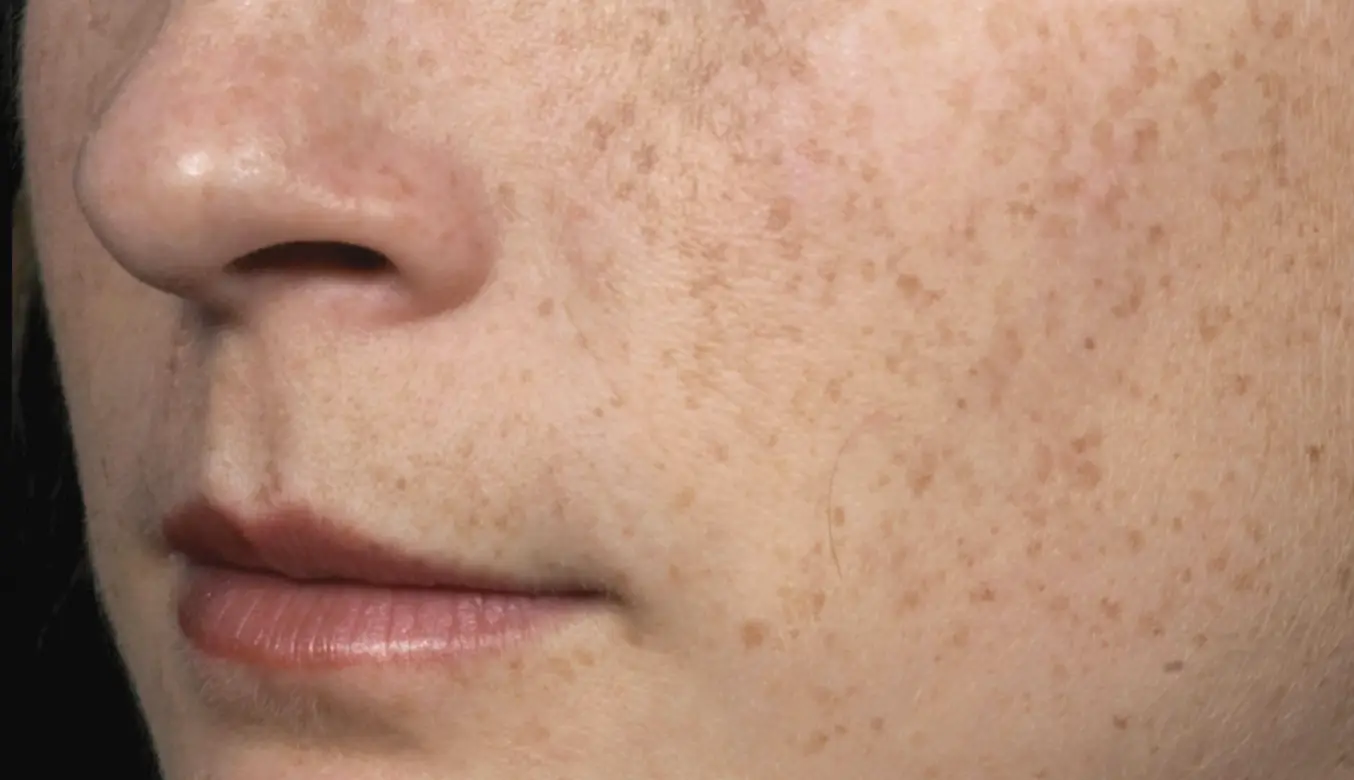
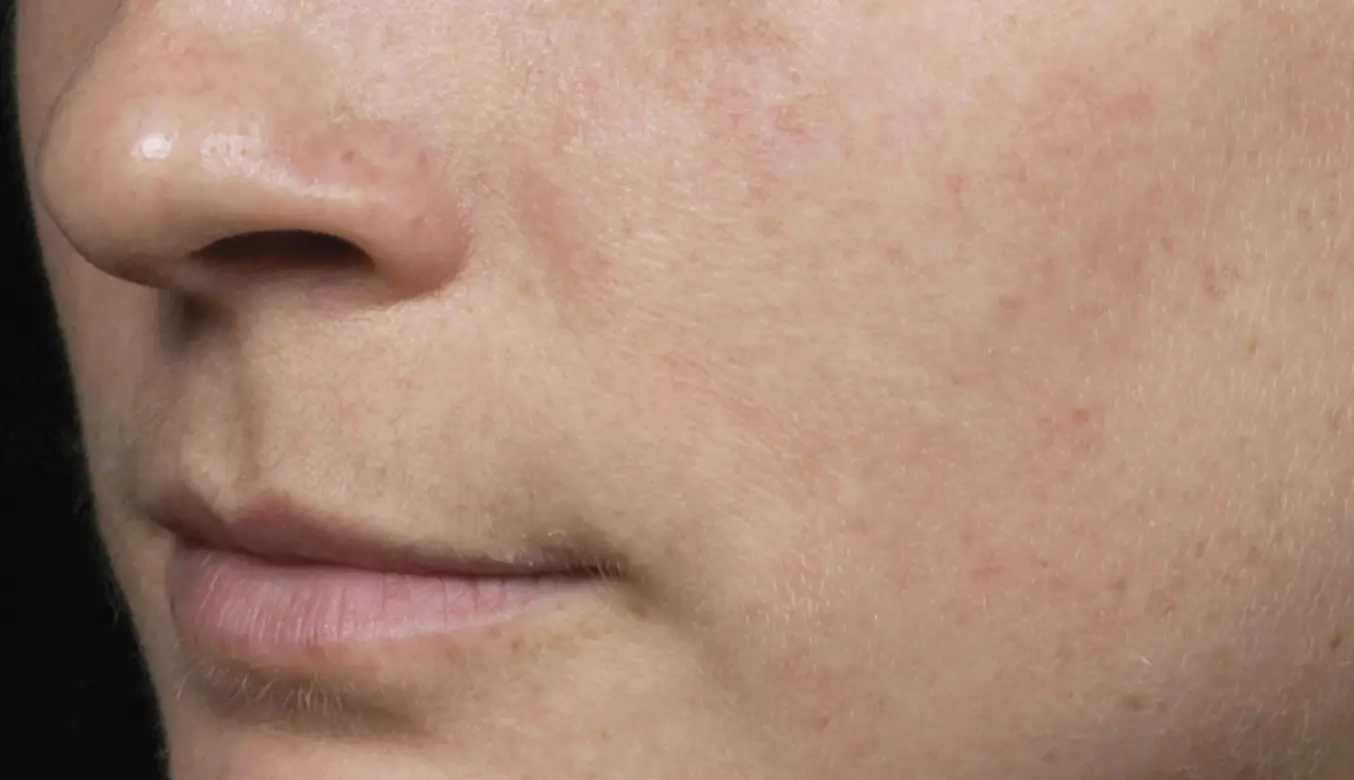
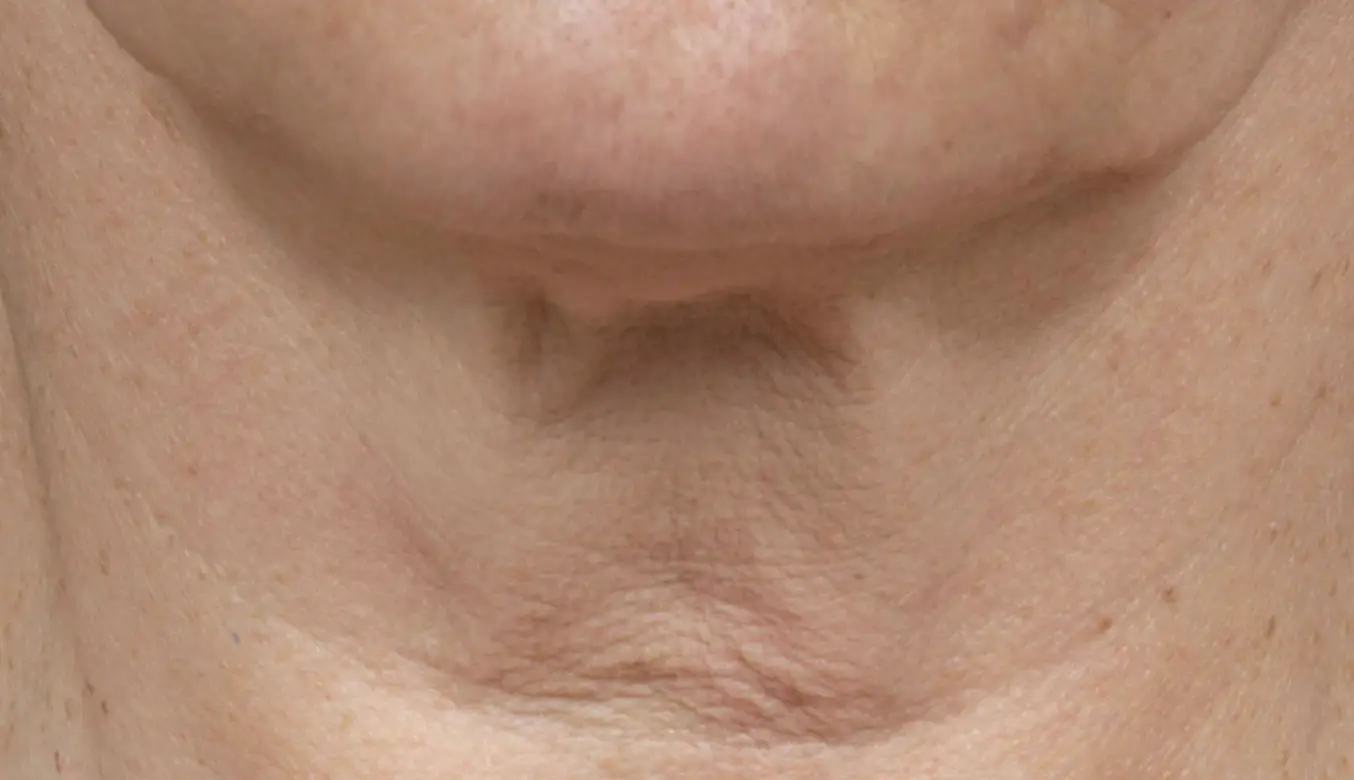
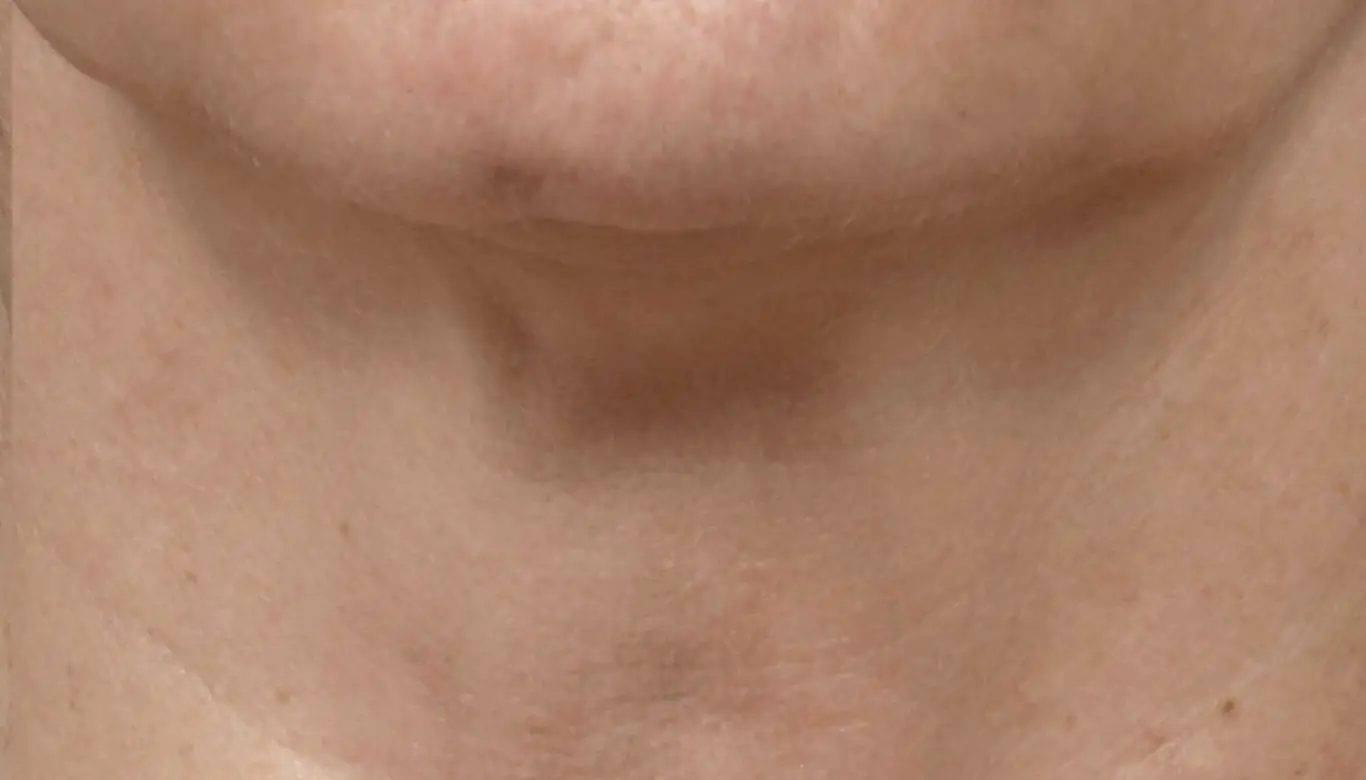
Introducing the latest and most advanced resurfacing technology from Solta.
Fraxel FTXTM builds on a legacy of award-winning performance designed to enhance speed and ease-of-use.
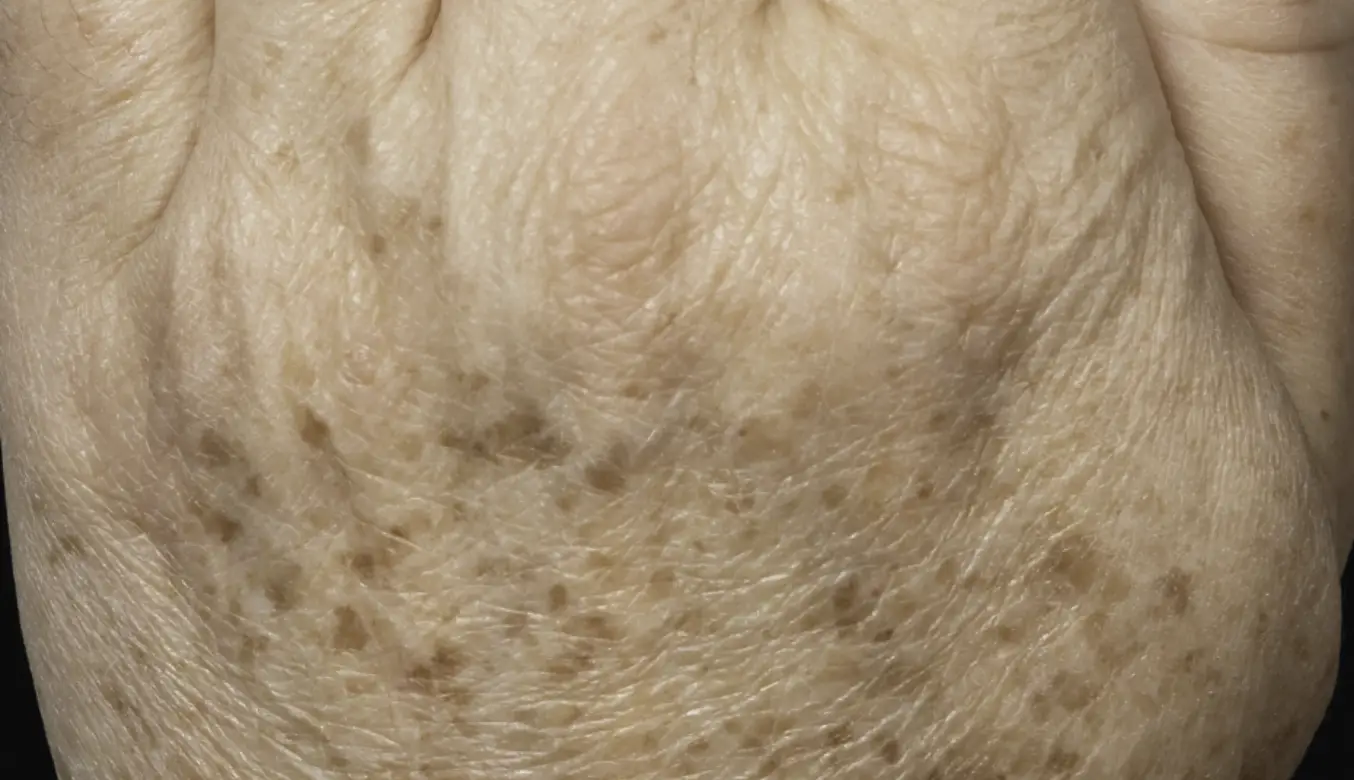
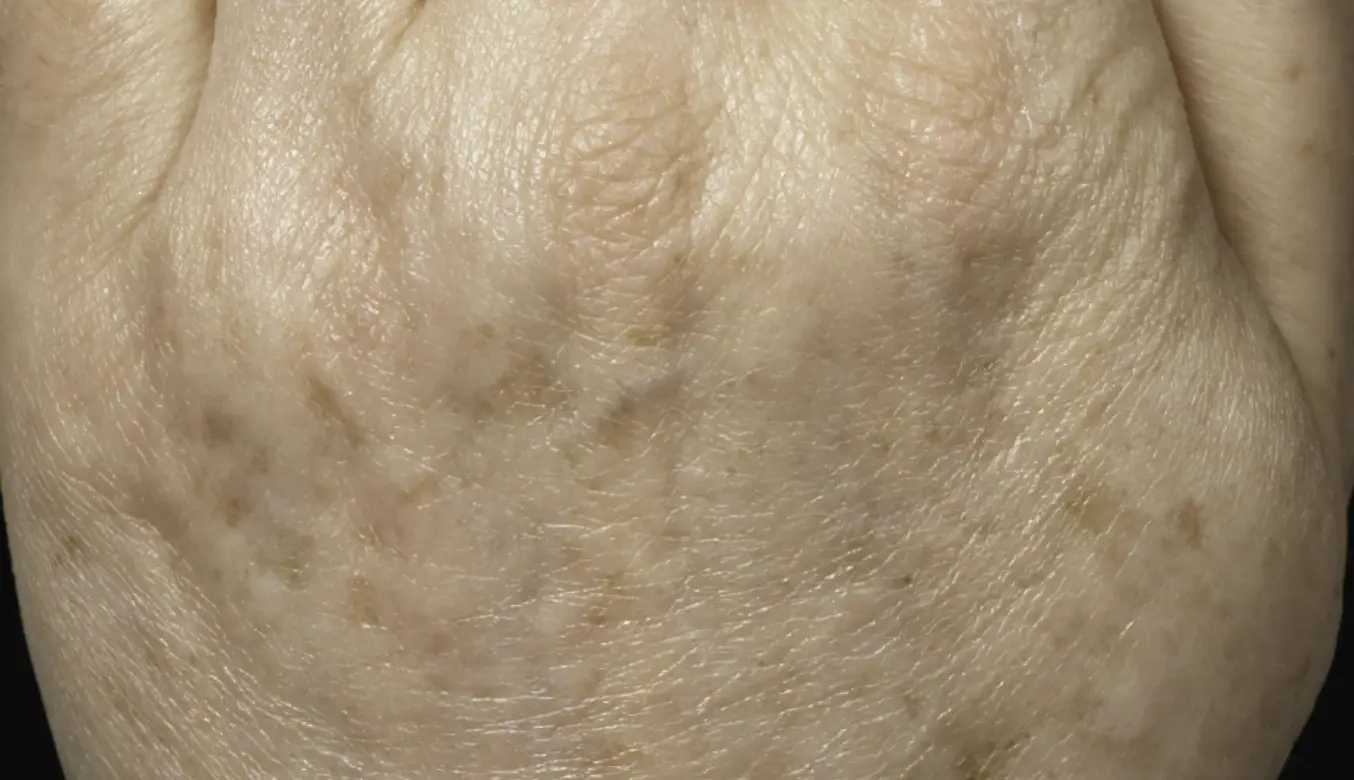
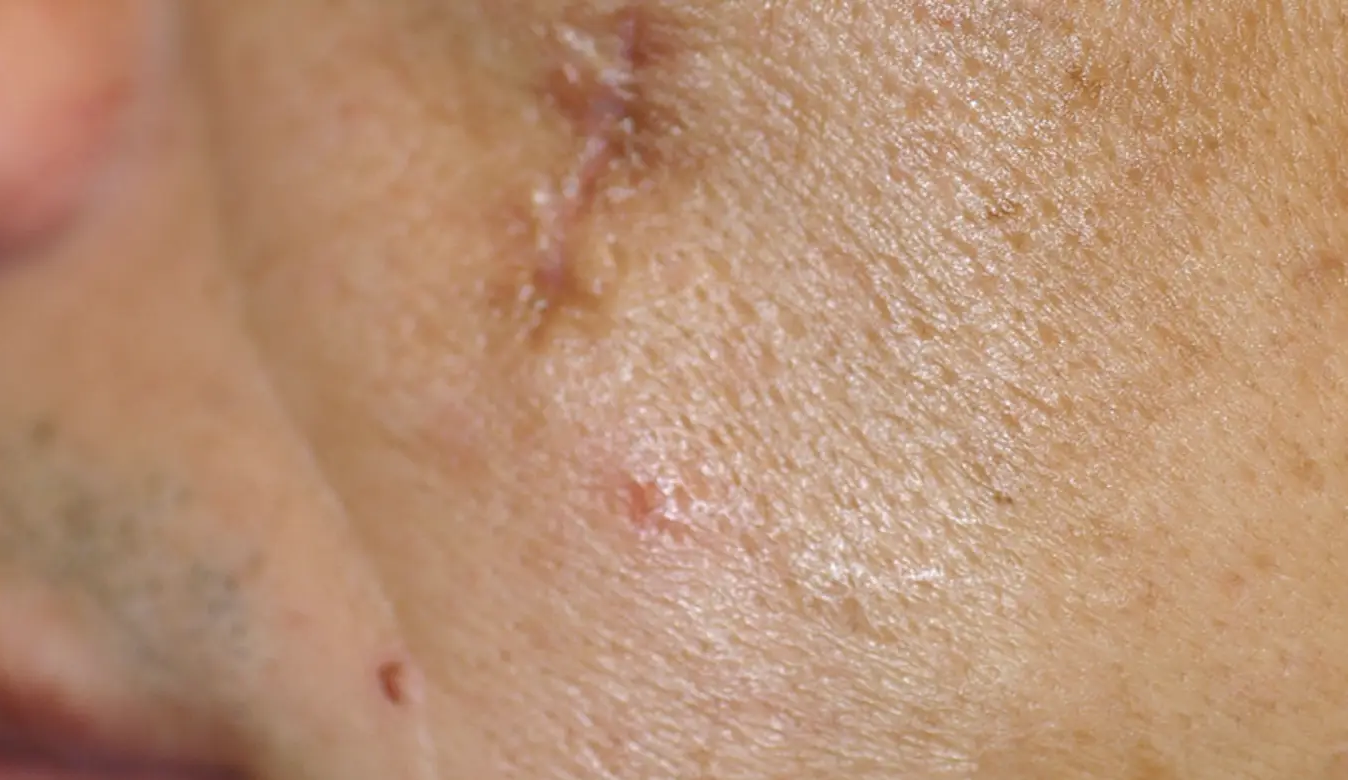
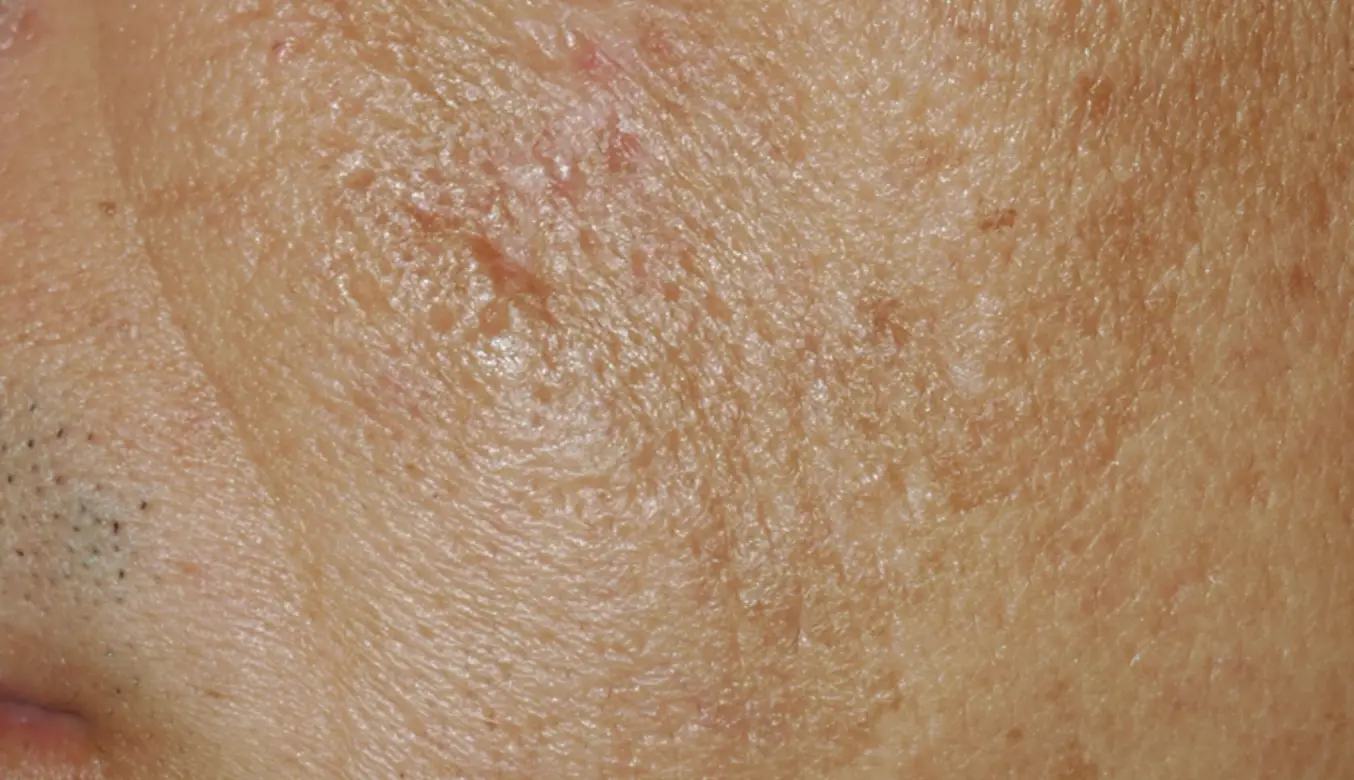
LEARN MORETREAT ALL SKIN TYPES ANYWHERE ON THE BODY
intelligent. Seamless. Proven.
- 1550 nm wavelength is an infrared Erbium fiber laser that can penetrate into the deeper dermis
- 1927 nm wavelength is an infrared Thulium fiber laser has a shallower depth of penetration, targeting the epidermal layer
- Intelligent Optical Tracking System™ is designed for consistent, uniform delivery of microscopic treatment zones with each laser pulse
- 2024 Shape “Best In-Office Treatment”
- 2024 NewBeauty “Best Resurfacing Laser”
Talk to one of our practice specialists to truly understand what Fraxel FTXTM can mean to your practice. We can answer questions about system flexibility, treatment efficacy, patient demand and of course, practice investment.